

It is also possible during this process, the work-energy and heat energy can be changed, but the temperature remains the same.
#Isothermal process workdone free
To keep the temperature constant, the system is free to change other parameters like pressure, volume, etc. Isothermal conditionsĪs my word, we know that the system’s temperature must stay constant in this isothermal process. If all the points on that surface are at the same temperature, then we can say that the surface is isothermal. Suppose we consider any surface flat, circular, or curvature, etc.
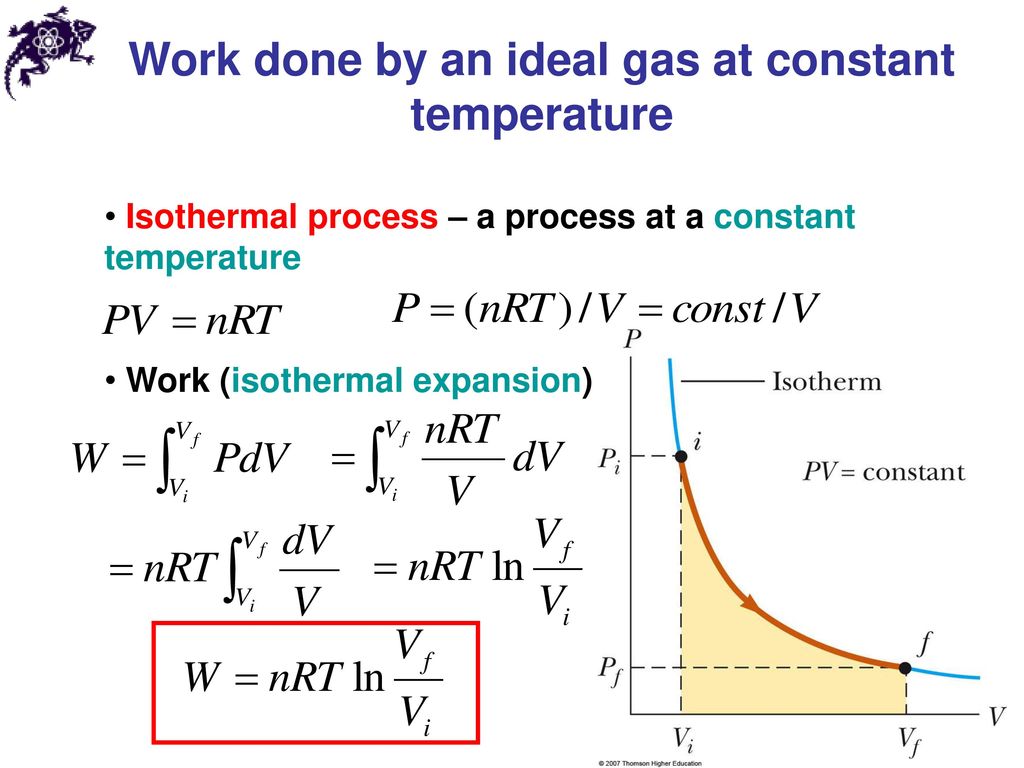
G is gravitational acceleration in m/s 2 Isothermal surface P1 & P2 are Pressures at Z1 & Z2, respectively, As we know from figure Vb is the final volume, and Va is the initial volume. We can consider that equation for the isothermal process. The above equation holds good for a closed system containing ideal gas. Now, here this is in isothermal process, so T = Constant, If we consider universal gas law, then the equation is given as below, This reaction is used in isothermal amplification techniques to amplify DNA.
#Isothermal process workdone full
The full form of PCR is a polymerase chain reaction. This situation is happening at the troposphere’s low level in various advection situations. It is defined as a vertical layer of air or gas with constant temperature throughout height. T is the temperature in kelvin Isothermal layerĪn isothermal layer term is used in atmospheric science. The Work done for this integral can be given as, We should consider the area under the curve A-B-VA-VB. If we want to write work done formula for it. We have used the PV diagram above paragraph.

Here, in both cases, the heat energy is removed and added, but the system’s temperature remains constant.Įxamples: Refrigerator, heat pump Isothermal work We can take the example of a refrigerator and heat pump. Isothermal is a process in which the system’s temperature remains unchanged or constant. Isothermal PV Diagram Credit Wikipedia Isothermal process example It is also known as the time-temperature- transformation diagram. Since then, around 1990, many isothermal amplification processes have been developed as alternatives to a polymerase chain reaction (PCR) Isothermal transformation diagramĪn isothermal transformation diagram is used to understand the kinetics of steel. Isothermal amplification of nucleic acids is a technique that is efficient and faster accumulating nucleic acid at the isothermal process. In this techniques, the DNA is amplified with keeping sensitivity higher than benchmark polymerase chain reaction (PCR) Isothermal nucleic acid amplification It is one of the techniques used for pathogen monitoring. Using isothermal calorimetry, one can find binding affinity, binding stoichiometry, and enthalpy changes between two or more molecules interactions. It is one technique to find thermodynamic parameters’ interaction in a chemical solution. The variation must happen at a very speedy.Some basic conditions for adiabatic are as below : The variation must be happening at a slow rate.The temperature should remain constant.Some conditions for an isothermal process are : This isothermal process is considered Isothermal compression. Let’s consider another condition if the piston is moving from TDC to BDC (Bottom dead center) with a constant temperature of the gas. Isothermal expansion is increasing volume with a constant temperature of the system. The temperature change is ΔT.įor the isothermal process, we can say that ΔT = 0 Isothermal expansion In this isothermal process, the system’s temperature remains constant throughout the process. Isothermal vs isentropic Isothermal definitionĪn isothermal process is a thermodynamic process.


 0 kommentar(er)
0 kommentar(er)
